Published on 24 Sep 2025 by rovertech
https://www.nature.com/articles/s41598-024-77177-0
Background
Myopia is recognized as a significant global public health concern and a leading cause of visual impairment1. Axial length (AL) elongation is a paramount factor in myopia progression, and measuring AL has been firmly established as a critical assessment in studying the progression and control of myopia. It is widely regarded as the gold standard for evaluating the effectiveness of myopia control treatments2–4. In clinical practice, AL measurement can be up to 10 times more sensitive in detecting myopia progression compared to relying solely on refraction5. This sensitivity is particularly crucial in clinical settings where cycloplegic refraction may not be feasible.
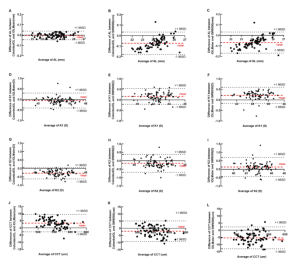
The Colombo IOL (Moptim, Shenzhen, China) is an emerging instrument that employs SD-OCT technology for the measurement of ocular biological parameters, including AL, central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness (LT), keratometry (K), white-to-white (WTW) distance, and pupil diameter (PD). The assessment of these indicators bears significant clinical implications. For instance, AL, CCT, K1, and K2 can be utilized to identify biomarkers associated with conditions such as keratoconus and pathological fundus changes associated with high myopia6. Currently, the available instruments for obtaining these data also encompass Swept Source OCT (IOLMaster 700, Carl Zeiss Meditec, Germany) or OLCR Biometer (SW9000, Suoer, Tianjin, China).
Despite the prevalent use of optical biometers, there is a notable gap in studies that specifically evaluate the SD-OCT biometer of the Colombo IOL. A recent publication has assessed the agreement of ocular parameters in adult patients with myopia between the Colombo IOL and the IOLmaster 7007, while limited research on children’s cases is available in current literature. Furthermore, the accuracy of the Colombo IOL has not been simultaneously compared with OLCR instruments (SW-9000). Consequently, the main objective of this study is to evaluate the accuracy of the Colombo IOL and establish its agreement with the IOLMaster 700 and SW-9000 in measuring essential parameters such as AL, CCT, K1, and K2 in healthy pediatric population.
https://www.nature.com/articles/s41598-024-77177-0/tables/2
Conclusions
In the case of pediatric subjects in a healthy state, the novel SD-OCT biometer demonstrated a strong agreement with the SS-OCT biometer in measuring AL, and CCT. This suggests that these two instruments can be effectively used interchangeably in AL in clinical practice. However, corneal refractive power could not be used interchangeably in clinical practice.